Learning Outcomes:
i. Understand the unique reactivity of benzene and its susceptibility to electrophilic addition reactions under specific conditions.
ii. Identify the factors that influence the regioselectivity of electrophilic addition reactions in benzene.
iii. Explain the mechanism of electrophilic hydrogenation of benzene and its applications.
iv. Describe the addition reactions of methylbenzene, including hydrogenation and Friedel-Crafts alkylation.
v. Appreciate the importance of understanding addition reactions in organic synthesis and industrial processes.
Introduction:
Benzene, the cornerstone of aromatic chemistry, is generally resistant to electrophilic addition reactions due to its exceptional stability arising from its delocalized pi electrons. However, under specific conditions, benzene can undergo addition reactions, where new atoms or groups are added to the benzene ring. This lesson delves into the addition reactions of benzene and its methylated derivative, methylbenzene.
i. Electrophilic Addition Reactions of Benzene: Overcoming Aromatic Stability
While benzene typically resists electrophilic addition reactions, it can undergo these reactions in the presence of strong Lewis acids and powerful electrophiles. These conditions disrupt the delocalization of pi electrons, making the benzene ring more susceptible to attack by electrophilic species.
ii. Factors Influencing Regioselectivity: Directing the Electrophile
The regioselectivity, or the preferred position of attack, in electrophilic addition reactions of benzene is influenced by the presence of substituents on the benzene ring. Electron-donating substituents (e.g., alkyl groups) direct electrophilic attack to the ortho and para positions, while electron-withdrawing substituents (e.g., halogens) direct attack to the meta position.
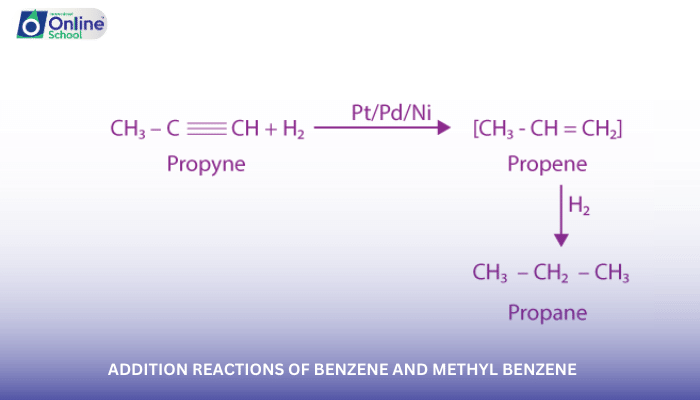
iii. Electrophilic Hydrogenation: Adding Hydrogen to Benzene
Electrophilic hydrogenation of benzene, also known as catalytic hydrogenation, involves the addition of hydrogen atoms to the benzene ring under high pressure and in the presence of a catalyst, such as nickel or platinum. This reaction produces cyclohexane, a saturated cyclic alkane.
iv. Applications of Electrophilic Hydrogenation
Electrophilic hydrogenation has significant applications in the chemical industry:
Hydrogenation of Fats and Oils: The process of hydrogenation converts unsaturated fats and oils into saturated fats and oils, altering their physical properties and shelf life.
Production of Cycloalkanes: Cycloalkanes, such as cyclohexane, are valuable intermediates in the synthesis of various organic compounds, including pharmaceuticals, plastics, and synthetic fibers.
v. Addition Reactions of Methylbenzene: Exploring Reactivity
Methylbenzene, also known as toluene, is a methylated derivative of benzene. It exhibits similar reactivity patterns to benzene, undergoing electrophilic addition reactions under specific conditions.
Hydrogenation of Methylbenzene: Methylbenzene can be hydrogenated to form methylcyclohexane under similar conditions as benzene.
Friedel-Crafts Alkylation: Friedel-Crafts alkylation involves the addition of alkyl groups to the benzene ring using a strong Lewis acid catalyst, such as aluminum chloride (AlCl3). Methylbenzene undergoes Friedel-Crafts alkylation to produce a variety of substituted methylbenzenes.
Addition reactions of benzene and methylbenzene, though less common than electrophilic substitution reactions, offer valuable synthetic routes for introducing new functional groups and modifying the structure of these aromatic compounds. Understanding these reactions is essential for organic synthesis, industrial processes, and comprehending the diverse chemistry of aromatic systems.